|
Expert Views :: Mirror, Mirror in the Environment... Considering the Hormone Activity of Chiral Environmental Chemicals Thomas E. Wiese What is a chiral chemical? Chiral chemicals are compounds that exist in two distinct forms that are identical in composition and connectivity, but different because they are exact mirror images of each other. In 2-dimensional stick figure representations, these mirror images might look the same. However, in 3-dimensions, one form of the chiral compound is the right handed version and one is the left handed version. The difference in this apparent sameness becomes very apparent when we start thinking about placing one of our left hand gloves on the right hand. The glove has the right number of fingers and a thumb, but it does not fit well on the wrong hand. These right and left hand versions of chiral chemicals are called enantiomers or optical isomers because they each bend polarized light in opposite directions. Common nomenclature for enantiomers include (+), (-) or D and L designations for how the compound bends light and the R or S designation based on absolute atom connectivity rules. A 50/50 mixture of enantiomers is called a racemate or racemic mix. Typical commercial synthesis process tend to produce a racemic mixture of the desired chiral chemical. So, are chiral chemicals in our environment? Yes! Chirality is actually critical to life. The proteins of living things are made only of L amino acids. The sugars we use for energy are in the D form. Why I bring the concept of chirality to this e.hormone commentary is the fact that environmental contaminants can also be chiral. About 25% of pesticides, some PCBs and many other pollutants are chiral. This means that they exist in the environment in two forms, the right and left hand versions. It is also interesting that some pharmaceuticals are chiral. Examples include: Prozac, Ibuprofen and Thalidomide to name a few. Our interest in the potential environmental impact of chiral chemicals should peak when we consider what is known about chiral drugs (1). Only one enantiomer of Prozac is active, one enantiomer of Ibuprofen is active and one enantiomer of Thalidomide is therapeutic while the other is teratogenic (causes limb malformations). Just as organisms are selective for one optical isomer of amino acids and carbohydrates, they also select between enantiomers of drugs. Due to this fact, many pharmaceutical companies are working hard to develop methods for producing only the active optical isomer of chiral drugs. To reduce pollution, many European countries are now requiring the use of only the active isomer of chiral pesticides. The classic case of chiral selectivity in environmental contaminants is the pesticide o,p’-DDT. In the mid 1970s, it was determined that the (-) enantiomer of o,p’-DDT was much more estrogenic than the (+) isomer (2). This means that the well characterized feminizing effects of DDT are due largely to only one optical isomer (3). In fact, the molecular model of DDT rotating on the e.hormone web page is the S(+) form of o,p’-DDT which is much less estrogenic than the R(-) enantiomer. The chiral selectivity of estrogen action has also been described for certain metabolites of the synthetic hormone DES (diethylstilbestrol) by Ken Korach’s laboratory (NIEHS, RTP, NC) (4). These studies characterized not only the enantioselective activation of estrogen receptor, but also what amino acids in the ligand binding site are responsible (5). In the last few years, my laboratory has formed a collaboration with Wayne Garrison at the US EPA’s National Exposure Research Lab in Athens, GA to look at the endocrine disruption potential of chiral pesticides. Included in these studies were: o,p’-DDT, o,p’-DDD, o,p’-dicofol, o,p’-methoxychlor, heptachlor, heptachlor epoxide, cis and trans-chlordane, a-HCH, dichlorprop, ruelene and metolachlor. Through a series of contracts, EPA did the chiral separation of the enantiomers and my lab performed in vitro determinations of hormone activity (estrogen, androgen and progesterone) of each individual enantiomer. A summary of our latest studies was presented in the “Chiral Pollutants: Enantioselectivity & Its Consequences” symposium at the recent SETAC meeting held in Baltimore, MD on November 11-15th 2001. Highlights include mechanistic details of the previously determined estrogen activity of the o,p’-DDT-R(-) enantiomer as well as the determination that the antiandrogen activity of o,p’-methoxychlor, ruelene, cis and trans-chlordane and heptchlor epoxide are all specific for only one optical isomer (6). Of particular interest is the observation that the racemic mix of ruelene has no observed androgenic activity due to the fact that the (-) enantiomer is an androgen agonist while the (+) enantiomer is an androgen antagonist. One cancels out the other when tested as an equal mixture (racemate). 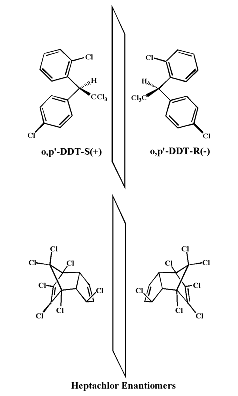 Examples
of chiral environmental chemicals showing both enantiomers reflected through a plane.
So, some chemicals in the environment are chiral and some of these can mimic natural hormones in specific ways. Why is this important and why should we look closer at these compounds? First, we must consider the environmental fate of each enantiomer of the chiral contaminants. Remember that degradation by microorganisms involves enzymes that may be enantioselective. Thus, one mirror image may be broken down quickly while the other persists in the environment. Since the population of bacteria and the enzymes they express depends on ecosystem conditions, the chiral selectivity of degradation may be variable from one contaminated site to another. Then we must think about the movement of these compounds into higher organisms. While passive absorption of both enantiomers will likely be similar, the exposure will depend on location. This means that the dose received by wildlife or humans could be a racemic mix (both enantiomers) or highly enriched in one enantiomer or the other. Simply looking for o,p’-DDT in tissues without resolving the optical isomers is not predictive of risk. Once the enantiomers of a chiral endocrine disrupter do get into an organism, we must then consider chiral selectivity in the metabolism and excretion of the compound as well as all the potential interactions each isomer may have with the multiple pathways of signaling between and within tissues and cells. Differences in these activities may be species and/or signaling mechanism specific and may involve one mirror image producing much more activity than the other. If metabolism is enantioselective, then one isomer may be quickly degraded and eliminated while the other persists in the organism. After metabolism, we must consider the toxicity of metabolites. Many hormone active agents require metabolism to produce toxic effects (e.g. methoxychlor (7)). Metabolic activation may also produce additional optically active isomers or remove chiral centers all together. Through oxidative metabolism, heptachlor is transformed into the endo or the exo-epoxide. So, in this one step, the two enantiomers of chiral heptachlor become four different epoxides. On the other hand, reductive metabolism of either o,p’-DDT enantiomer eventually produces the same non-chiral product, o,p’-DDE which has little estrogen activity, but is a much better antiandrogen than either o,p’-DDT enantiomer. Both the strongly estrogenic and the weakly estrogenic enantiomers of o,p’-DDT end up as one antiandrogenic compound. Now let us focus on the potential signaling disruption events within a cell or organism. If any of these processes are enantioselective for any given chiral toxicant, then depending on where exposure takes place, how metabolism occurs in the environment and within the organism, toxic effects may or may not be observed. Consider two birds exposed to the same amount of a chiral pesticide like heptachlor. One bird resides in a marshy area along Lake Huron and the other lives in a bayou in Louisiana. These are different ecosystems with different microbial populations. The racemic heptachlor may be degraded differently in each location, resulting in the persistence of a different enantiomer which then translates into different exposures for each bird. Once inside the bird, the two different enantiomers may act on distinct or even opposite signaling pathways. Thus, the same chiral compound in different environments might lead to completely different toxic effects. When we then add all the potential species specific responses to the chiral components, the picture becomes more complex. The toxic result may be completely different for other exposed organisms in these two environments. Finally, we must look closer into the receptor and gene specific effects of these mirror images of chiral environmental toxicants. It is easy to determine the estrogen or androgen receptor binding capacity of hormone active agents. What about the difference between enantiomers? Some may bind a receptor the same as their mirror image and some may have completely different specificity. If both enantiomers do bind the target receptor, what about the structural and functional changes that binding event induces? Will a nuclear transcription factor regulate the same genes in the same way when activated by the left handed version of a pesticide as when activated by the right handed isomer? What about enantioselective effects on the aryl hydrocarbon (dioxin) receptor function and it’s subsequent induction of metabolic function and capacity? The enantio-specific molecular interactions of chiral environmental agents are largely unknown. So, chiral chemicals such as amino acids and carbohydrates have been a critical component of the complex evolutionary process that refined all living things on our planet. For the last half a century, we have been introducing chiral contaminants into our environment. How the right and left handed versions of these chemicals are differentially effecting organisms and ecosystems is not clear. Within this uncertainty, two things are clear: Some enzymes and receptors will be regulated by certain enantiomers better than others and the resulting signaling events will translate into unique effects within organisms. One last thought. Consider the time in the future when we finally contact carbon based life on another world. While we would certainly expect to find differences between our intellect and politics, there may also be more fundamental differences due strictly to opposite evolutionary selections in the chiral preferences of our biochemistry. It will be interesting to compare historical notes on how each of our environments has tolerated chiral pollutants. Chances are good that our enantioselectivity for toxic chemicals will be different. References
|