Endocrine Disruption
|
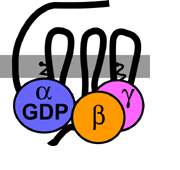
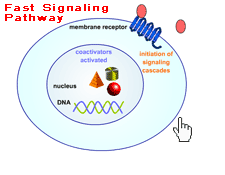
Actions : Docking :: Receptor Binding Steroid Receptors On the Cell Surface Surface receptors are embedded in a cell's outer membrane. Hormones binding to receptors nestled in the membrane can initiate gene expression or spark molecular signal cascades - lightening fast chain reactions of chemical communication inside the cell that immediately change cell functions. Cells respond to genetic expression in hours to days whereas the response to the membrane-bound steroid receptor’s nongenomic signals occurs very rapidly, usually in just seconds to minutes. The complex, cascading signal begins when hormones, or other signaling messengers, outside the cell bind with and activate certain cell surface receptors. The receptors, in turn, interact with special signaling molecules inside the cell called intracellular second messengers, including guanylyl nucleotide regulatory proteins (G proteins), inositol triphosphate, and cyclic adenosine monophosphate (cAMP). The second messengers then switch on the kinase enzymes. These specialized proteins phosphorylate - add phosphorus to - other enzymes. Adding phosphorous turns on many different kinds of cell enzymes that regulate a variety of responses including cell growth, electrolyte movement across the cell membrane, and hormone secretion. G Proteins
CAPTION: G proteins, found on the inside surface of the cell membrane, turn other proteins off and on.
CREDIT: Tulane University. G Protein-Coupled Receptors Three kinds of receptors with different molecular structures interact with steroid hormones at the cell surface. Two of the receptors, the G protein-coupled receptors (GPRs) and the G protein-coupled receptor-like receptors, are only found in the cell’s plasma membrane. The third is simply a nuclear steroid receptor tightly lodged in the cell membrane (Cato et al. 2002; Hammes 2003; Toran-Allerand 2004; Zhu et al. 2003a, 2003b). The receptors transverse the membrane and are situated with one end poking to the outside of the cell - where ligand binding occurs - while the other is positioned inside the cell - where the signal is transmitted. GPRs and their ilk possess the unique property of being able to bind with steroid hormones located outside the cell to initiate and promote fast, nongenetic hormone-like changes inside the cell. Prior to their discovery, how cells without steroid nuclear hormone receptors reacted to hormones perplexed researchers. Prime examples are certain breast cancer cells that lack the nuclear estrogen receptors ERalpha and ERbeta but still grow in response to the estrogen 17-beta estradiol (E2). Why the cells respond to the hormone was a mystery until an estrogen-specific G protein-coupled receptor called GPR30 was identified in the plasma membrane of these cells. When E2 binds to GPR30, a cascade of enzyme activation turns on cell growth (Cato et al. 2002). Another family of membrane receptors with GPR-like molecular structures binds with and responds to progestin hormones. The progestin membrane receptors (mPRs) trigger internal signaling pathways similar to GPRs rather than invoking gene expression typical of steroid hormone receptors. Like GPRs, these receptors have seven trans-membrane domains. Pertussis toxin, a bacterial poison that prevents certain G proteins from interacting with other second messengers inside the cell, can block their action. However, the receptors are probably an entirely new class of membrane receptor since their amino acid sequence differs from other GPRs. Researchers first found mPRs in spotted seatrout and deciphered their important role in stimulating fish egg maturation. The complicated series of signals begins when the progestin hormone progesterone binds mPR and activates Gai protein. Gai inhibits activity of the second messenger adenylate cyclase (an enzyme that converts ATP to cAMP and pyrophosphate), decreasing intracellular cAMP levels and reducing protein kinase A (PKA) activity. Lower PKA activity releases phosphatase Cdc25C to dephosphorylate and activate the cell cycle proteins Cdc2 and cyclin B. Active Cdc2 and cyclin B are necessary for germinal vesicle breakdown (dissolution of the nucleus) and final oocyte (immature egg) maturation (Zhu et al. 2003a, 2003b).
GPR-like mPRs have been discovered in a variety of backboned animals, including several kinds of fish, in frogs and mammals (pigs, mice), and in human tissues in the reproductive tract, kidneys, intestine, and brain. One type of mPR rouses both human and fish sperm, making the sperm competent to fertilize eggs (Zhu et al. 2003a). The mPR in fish sperm, though, can be blocked by foreign chemicals, particularly the organochlorine insecticide kepone and o,p’-DDD, a metabolite of the insecticide o,p’-DDT (Das and Thomas 1999). back to topReferences
back to top |