Endocrine Disruption
|
The Hormones : Estrogens
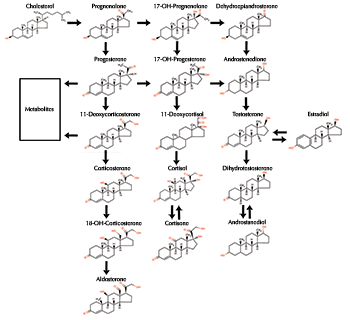
In humans and other vertebrates, estrogens are made primarily in the female ovaries and in small amounts in the male testes and the adrenal glands, brain, and fat of both sexes. 17-beta estradiol is the most abundant and potent natural estrogen in all vertebrates. Estrone and estriol are other types. Estrogen-like chemicals play a poorly understood role in the reproductive cycle of some invertebrates, including mollusks and corals (Di Cosmo et al. 2001; Tarrant et al. 1999) Birth control, hormone replacement therapies (HRT), cancer drugs, and other pharmaceuticals contain synthetic estrogens. Some, like ethinyl estradiol, are used alone or combined with artificial progesterone-like hormones in birth control. In HRT, natural and synthetic estrogens with or without artificial progesterone control menopause symptoms such as hot flashes, skin and vaginal dryness, and bone loss. However, higher risks of breast cancer, heart attack, blood clots, and other serious threats temper the benefits (Women's Health Initiative Steering Committee 2004; Writing Group for the Women's Health Initiative Investigators 2002). Women can tailor treatments by weighing known risks and benefits.
Like all steroid hormones, estrogens produce effects by docking with receptors on the cell's membrane surface or inside the cell in the liquid cytoplasm. Receptor binding triggers different chemical signaling systems depending on receptor location. An estrogen uniting with a surface receptor starts a lightening-fast chemical relay in the cytoplasm that can trigger nitric oxide production, flood the cell with calcium, or initiate hormone release. Effects Estrogens have wide-ranging effects throughout life and in both sexes. Most backboned animals depend on the hormones to control and regulate female development, reproduction, and sex characteristics. Their signals also affect blood fat levels, enzyme production, water and salt balance, bone density and strength, skin and blood vessel elasticity, heart muscle, and brain functions such as memory and sexual and maternal behavior. In the developing animal, estrogens guide formation of the female reproductive tract - vagina, uterus, ovaries - and external genitals. At puberty, their actions guide the appearance of secondary sex characteristics, such as breast and body hair growth and fat distribution in humans and mammary gland and nipple growth in other mammals. The adolescent bone-growth spurt is fueled, and then halted, by estrogens. Estrogens secreted from the ovaries fluctuate during the reproductive cycle, which may occur episodically (frogs mating in response to rainfall), bi-weekly (many marine animals), monthly (humans), semi-annually (cattle), or even bi-annually (elephants). Estrogen levels rise in response to internal or external cues (temperature, moon phase, light cycle, presence of potential mates), stimulating eggs to move from their suspended state since birth to finish maturing. Because the nursery cells surrounding the eggs make estrogens, hormone levels peak at ovulation, when the ovary releases the egg or eggs. Afterward, levels fall rapidly to restart the cycle. When the human ovaries enter menopause, estrogens stop fluctuating cyclically and fall to low levels. The adrenal glands and other tissues that convert androgens to estrogens then become the prime, steady source of the hormones. Males need estrogens, too. The hormones influence fertility through the prostate, testis, and other sex tissue and by controlling fluid absorption - too much dilutes sperm and decreases egg fertilization - around the maturing sperm in the epididymis, a coiled structure beneath the testes that stores and transmits sperm (Hess et al. 1997). At puberty, estradiol regulates growth hormone and determines final height, as in females, by shutting off bone growth at the growing ends, or ephiphysis, of arm and leg bones. Researchers discovered this off switch after examining a still growing 6-foot-8-inch man with an estrogen receptor gene mutation (Smith et al. 1994). Estrogens can also harm people and animals. Long-term exposure to the hormones can increase the risks of breast, endometrial, and vaginal cancers in women (US Department of Health and Human Services 2002). Too much estrogen aggravates endometriosis, a painful growth of the uterine lining outside the uterus, while too little estrogen weakens bones (osteoporosis). Males exposed at certain times during development can develop female features and organs (feminized) or have diminished male characteristics (demasculinized) that might lead to testicular cancer, reduced sperm health, or genital defects, such as a malformed penis (hypospadias) or undescended testicles (cryptorchidism). back to topEstrogen Disrupters Estrogen mimics, blockers, or other interlopers are the best known and studied of all endocrine disrupters (the chemical or physical agents that alter hormone production or function). Guilty substances include natural plant compounds called phytohormones, heavy metals, and synthetic chemicals, such as drugs (DES), pesticides (DDT), persistent organochlorine pollutants (PCBs), and industrial chemicals (phthalates, bisphenol A). Food, household products, medical devices, and water may contain pseudohormones that get into the bodies of animals and people. Once there, these saboteurs can interfere in a number of ways, even though they vary widely in appearance, receptor binding strength, and potencies (the ability to produce an effect). Most vulnerable are developing embryos, infants, and very young animals, but adults are also susceptible. Effects, documented in humans, wildlife, livestock, laboratory animals, and cultured cells, run the gamut of reproductive cancers, behavioral changes, and reproductive problems. For instance, plant estrogens in clover and a potent fungal toxin found in contaminated grain both block sheep reproduction. Fish living below sewage treatment plants have hormone imbalances, and males may have both testes and ovaries (intersex). And long-lived pollutants such as DDT and dioxins may contribute to abnormal parenting behavior in gulls. click here to read more about wildlife effects... Humans may face exposure risks, too. Phytoestrogens in soy and legumes, such as genistein and coumestrol, bind the estrogen receptor and may both aggravate or protect against human breast and prostate cancers. Some daughters born to women who took the strong, synthetic estrogen diethylstilbestrol (DES) during pregnancy developed rare vaginal cancer and suffered with infertility later in life. Sons, too, were affected and have higher rates of noncancerous epididymal cysts than unexposed men (Gill et al. 1979). Grandchildren may also face health issues. Rodent research finds multi-generational health problems in both granddaughters and grandsons of those treated with DES (Newbold et al. 1998. 2000). click here to read more about human effects... click here to read more about phytoestrogens... While no clear links are yet evident, some believe increasing exposure to estrogen-like chemical mixtures in everyday life contributes to rising rates of testicular and breast cancers, increasing numbers of sex organ deformities, declining sperm counts, and precocious puberty in industrialized countries. Research History Charles R. Stockard and George N. Papanicolaou first described estrogens' actions in guinea pigs in 1917 (Stockard and Papanicolaou 1917). In 1922, Joseph A. Long and Herbert M. Evans followed with similar findings in rats (Long and Evans 1922). Both groups observed that ovarian follicle swelling, prior to the egg leaving the ovary (ovulation), was followed by uterine lining growth and vaginal cell maturation. These discoveries were the first to explain tissue changes during the menstrual cycle and their relationship to pregnancy (Hadley 2000). Later, Edgar Allen and Edward A. Doisy isolated the responsible steroid hormone, called it estrone, and described a test to detect this estrogenic activity in biological samples (Allen and Doisy 1923). Since then, their test, or similar ones, has become the standard way to detect, identify, and characterize natural and synthetic compounds with estrogenic activity (Lieberman 1996). Generally, any natural steroids, plant compounds, or synthetic chemicals are considered estrogenic if, in laboratory tests, they bind to estrogen receptor and promote cell division, induce cell growth, or produce certain proteins in female sex tissue (breast or uterus) (Hertz 1985). In the early 1920s, Bernard Zondek showed that willow tree flowers mimicked estrogen, confirming the existence of phytohormones (Hertz 1985). The search for hormones and phytoestrogens drove mid-20th century research that first isolated, then reproduced, the natural estrogens, progestins, and androgens. Commercial prospects for supplements or contraceptives fueled production of DES (1938), ethinyl estradiol (1950s), and other synthetic estrogens. More recent genetic and molecular research is unraveling the complex interplay among hormones and their signaling partners, including the discovery of a second estrogen receptor, ER-beta (Kuiper et al. 1996), and specialized roles of membrane receptors (Hewitt et al. 2005). back to topReferences
back to top |