Endocrine Disruption
|
The Hormones : Progestins
Progestin hormones prepare, sustain, and build. With them, pregnancy, some behaviors, and sex hormones endure. Indeed, progestins are nicknamed the pregnancy hormones because they prep for and maintain the body during pregnancy. Their essential role in egg and sperm maturation, sexual receptiveness, and estrogen production are lesser known. Not surprisingly, females make and use more progestins than males.
CAPTION: Progesterone, the best known progestin hormone, is nicknamed the pregnancy hormone. (click image to manipulate). CREDIT: PubChem, National Library of Medicine. Construction and Production
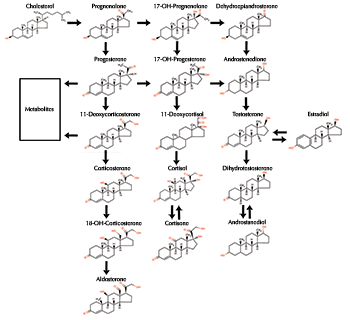
In humans and other vertebrates, progestins are made primarily in the female ovaries and male testes. Progesterone is the most abundant and potent progestin in four-legged vertebrates. 17-alpha-hydroxyprogesterone and 10-alpha-hydroxyprogesterone are weaker forms found in humans. The most potent progestins in fish are 17-alpha-20-beta-dihydroxyprogesterone and 17-alpha-20-beta-21-trihydroxyprogesterone. These hormones trigger the process of final maturation in fish eggs and sperm. Many invertebrates can synthesize progesterone but it is not known if it functions as a hormone in these animals. Birth control, hormone replacement therapies, cancer drugs, and other pharmaceuticals contain synthetic progestins. These manufactured hormones may not produce the same broad actions as natural progestins and can sometimes counteract androgens giving men female characteristics (as can natural progesterone). Some progestins used alone or in combination with synthetic estrogens in contraceptives are norethindrone, norgestrel, and ethynodiol diacetate. Artificial progestins such as medroxyprogesterone acetate and norethindrone are used in breast and endometrial cancer treatment. Revving Up
Like all steroid hormones, progestins produce effects by docking with receptors on the cell's membrane surface or inside the cell in the liquid cytoplasm. Receptor binding triggers different chemical signaling systems depending on receptor location. A steroid hormone uniting with a surface receptor starts a lightening-fast chemical relay in the cytoplasm that changes cell chemistry and initiates hormone release, nudges the cell from the resting to growth phase of its life cycle, or changes the cell’s energy use. In contrast, when steroid hormones go inside a cell, they can unite with a receptor to form a hormone/receptor unit that moves into the nucleus, attaches directly to special DNA binding sites, and activates protein-producing genes. The proteins drive the cell changes guiding progestin-controlled growth and development (Cato et al. 2002).back to top Effects Progestins have broad effects in backboned animals. One critical role is as a building block for all other steroid hormones, including androgens and estrogens. In mammals, they prepare for and sustain pregnancy. In fact, progestins were originally defined as steroid hormones that prepare the uterus for the fertilized egg to implant. Progesterone, the best-known progestin, fluctuates throughout life to support vital developmental and reproductive functions in women. The hormone assists with breast development and function during puberty, menstrual cycles, and pregnancy. A hormone trio - progesterone, estrogen, and prolactin - controls the rapid cell division and duct expansion associated with breast growth in early life. Then, every month after a women’s first menstrual cycle, progesterone readies the mammary glands for pregnancy and continues to guide tissue growth and prevent milk release during pregnancy. The hormone may play a role in breast cancer risk due to its life-long association with breast development. 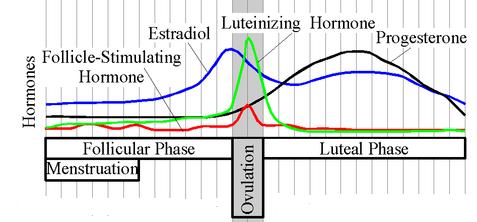
CAPTION: Progesterone levels fluctuate through a women’s monthly cycle. CREDIT: Wikipedia. In the reproductive years, secreted levels follow the rhythm of the monthly cycle. The ovaries make and release large amounts immediately after ovulation (release of the egg from the ovary) to prepare the uterus for the fertilized egg and potential pregnancy. If no fertilized egg attaches, then no pregnancy occurs. Progesterone concentrations fall, reaching the lowest levels after menstruation, and begin climbing again prior to ovulation. During and after menopause, the adrenal gland makes the hormone and levels stabilize and drop to about a third of those in young women. Progestins play a critical role in the reproductive cycle of all male and female vertebrates, preparing eggs and sperm for fertilization. In many animals, progestins act on the brain and are involved in mating and parental care behaviors of both sexes. Sexual receptivity in rodents and nest building and brooding in birds and fish are controlled by progestins. back to top Progesterone Disrupters Some natural plant compounds can mimic progesterone. Steroids called saponins and other related molecules in certain herbs produce progesterone-like effects by attaching to the progesterone receptor or interrupting hormone signaling pathways. The wild yam (Dioscorea villosa), chasteberry (Vitex agnus castus), and guggal (Commiphora mukul) contain known progesterone mimics and interferers. These medicinal herbs are taken to ease premenstrual syndrome and menopause symptoms (Memorial Sloan-Kettering Cancer Center 2005). Additionally, synthetic chemicals can cancel out progestins' effects in animals. These antiprogestins bind to progesterone receptors or interrupt progestin-controlled processes. In lab studies, the pesticides alachlor, endosulfan, and kepone interfere with progesterone binding to the alligator progesterone receptor (Vonier et al. 1996). In other studies low doses of kepone and o,p'-DDD bind progesterone receptors on fish eggs and impede egg maturation in spotted seatrout and Atlantic croaker (Das and Thomas 1999). Methoxychlor interferes in a different way to cause similar egg maturation problems in African clawed frogs. The chlorinated insecticide derails the normal chemical relay started after progesterone attaches to its receptor (Pickford and Morris 1999). click here to read more about wildlife effects... click here to read more about human effects... Other agents change the way hormones are built to both enhance and reduce progesterone levels, depending on dose and place of action. Cadmium, a ubiquitous pollutant used in nickel/cadmium (NiCad) batteries, some paints, and vehicle brakes, and a major part of tobacco smoke, increases ovarian progesterone production at low levels but lowers it at higher doses (Henson and Chedrese 2004). The heavy metal may either aid or inhibit the enzymes that convert cholesterol to progesterone, depending upon cadmium concentrations. During human pregnancy, a mother's cadmium exposure is linked to low birth weight and increased risk of miscarriage. Tissue culture experiments show that cadmium compromises the placenta’s ability to make progesterone. Insufficient progesterone levels can contribute to the risk of miscarriage and premature delivery (Henson and Chedrese 2004). Research History Progesterone was first isolated and artificially made in 1934 (Butenandt and Westphal 1934; Hadley 2000). By 1944, chemists were trying to create a synthetic progestin to prevent ovulation as a form of birth control. In 1949, Russel Marker learned about an inexpensive source of sapogenins - a plant compound that could be converted to synthetic progestins - in barbasco, the wild Mexican yam. Norethindrone, a synthetic testosterone capable of blocking ovulation, was manufactured in 1951 and efforts to produce oral contraceptives for women accelerated. During the 1950s and 1960s, chemists raced to make effective and cheap progesterone mimics. Early versions of "the pill" contained extremely high doses of synthetic estrogen (ethinylestradiol) and progestins. Current versions contain low doses of ethinylestradiol-progestin mixtures or progestin alone and are generally considered safe for nonsmokers with normal blood pressure (Djerassi 1966; Glasier 2002; Schiebinger 2001). back to topReferences
back to top |