Endocrine Disruption
|
The Hormones : Thyroid Thyroid hormones support nearly every body system. In humans and other backboned animals, the iodine-containing hormones guarantee proper development of the brain, skeleton, and organs. Yet, too much or too little derails the body's delicate balance causing goiter and other health troubles. These distinct regulators work alone or in cooperation with other hormones to generate energy, control cell oxygen use, and moderate many other life processes in both males and females.
Construction and Production
CAPTION: The two most active thyroid hormones, triiodothyronine (T3) and thyroxine (T33), contain iodine atoms typical of the thyroid hormones. (click image to manipulate). CREDIT: PubChem, National Library of Medicine. Thyroid hormones are a group of chemically similar substances with a unique iodine-amino acid structure. Food and water supply the needed element iodine. Its presence in biologically-active molecules is unusual and distinguishes the thyroid hormones from other chemical messengers. Amino acids are special molecules that combine to form proteins. Humans have 20 different kinds of these water-soluble building blocks.
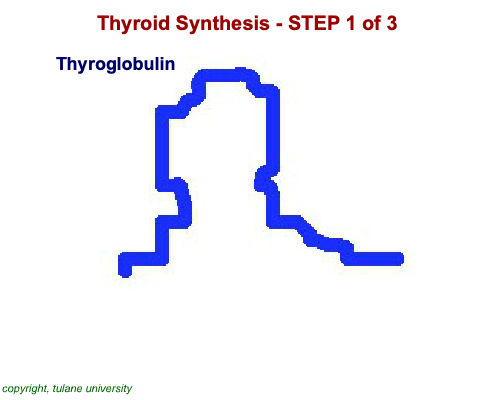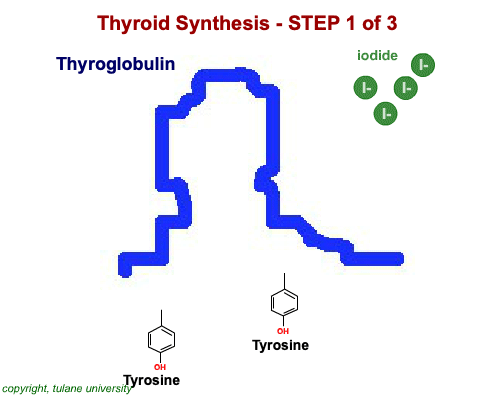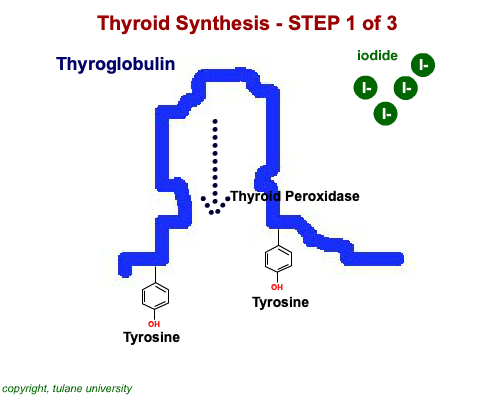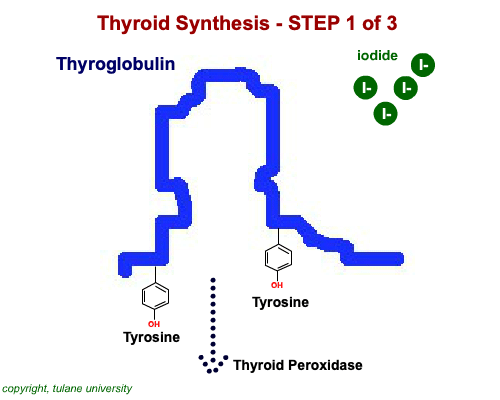
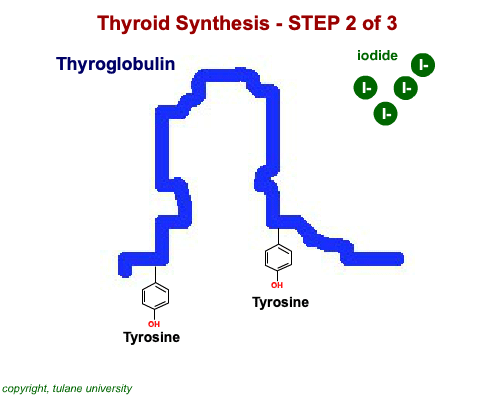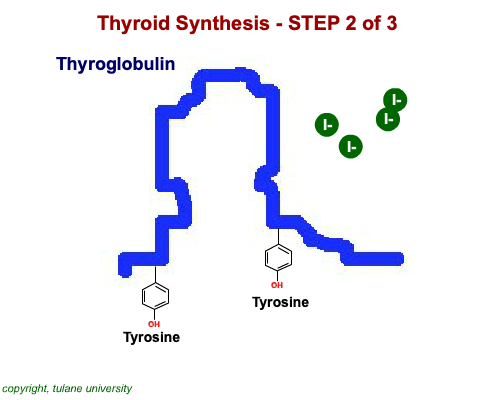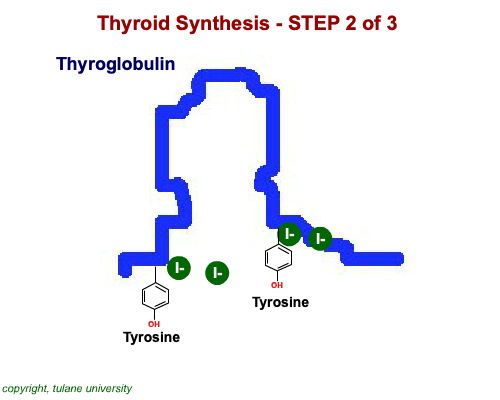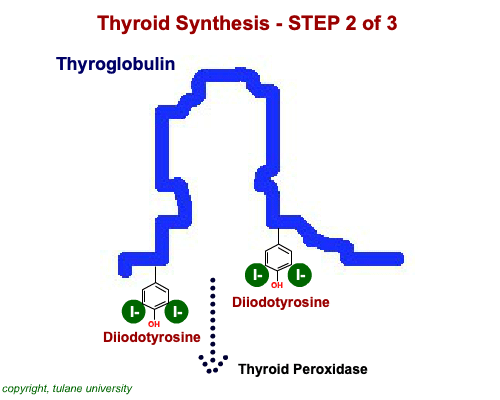
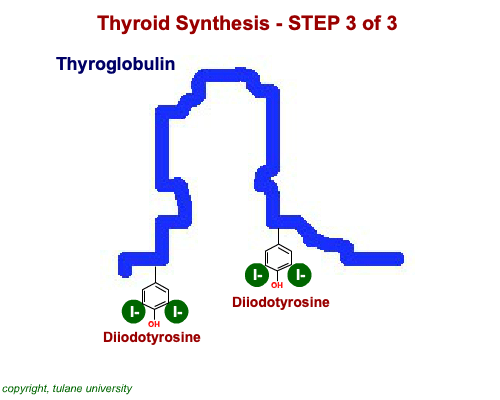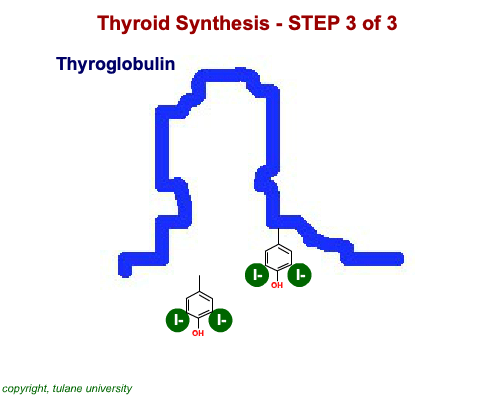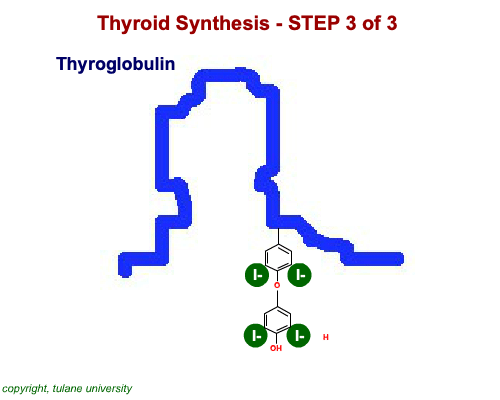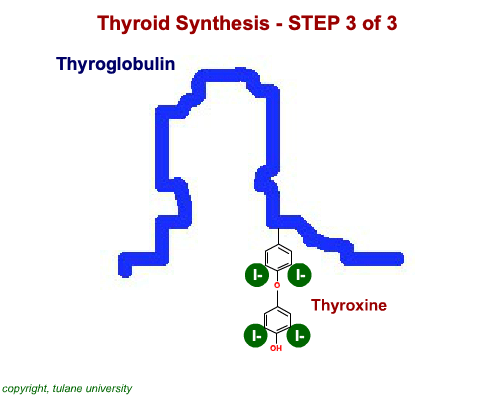
The most common thyroid hormones are made in the vertebrate thyroid gland. The complex building process begins with a long chain of linked tyrosine amino acids called thyroglobulin.One or two iodine atoms are first added to each tyrosine. Then, pairs of the iodinated amino acids are clipped from the thyroglobulin chain creating mainly thyroxine and some triiodothyronine. Thyroxine, the most abundant thyroid hormone, contains four iodine atoms and is nicknamed T4, while triiodothyronine, the most active form, has three and is referred to as T3. Once made, the thyroid gland releases the hormones into the bloodstream where protein chaperones, called thyroid transport proteins, accompany them to target cells in tissues all over the body. In the target cells, enzymes remove one of thyroxine's four iodine atoms converting the hormone into the highly active triiodothyronine. androgen in four-legged vertebrates, it is the most potent variety in bony fishes and sharks. Revving Up
Thyroid hormones produce effects by docking with protein receptors in thyroid-sensitive tissues. The hormones can bind with receptors on the cell's membrane surface and inside the cell on the mitochondria or in the nucleus. Binding activates chemical processes and protein producing genes that control a cell's energy and metabolic functions (Cato et al. 2002). Thyroid hormones usually take many hours to days to achieve their final effects. back to topEffects Almost all body functions carried out in nearly every tissue rely on thyroid hormones. Their actions and influence are so wide-ranging that vertebrates cannot live without them. Among other things, thyroid hormones specifically affect brain development; heart rate; lung function; blood function; bone growth; steroid hormone production and breakdown; sugar, fat, and protein breakdown; and some immune processes. Whether alone or in concert with other hormones, the indispensable messengers regulate life-sustaining processes essential for normal growth, development, reproduction, and even behavior. For example, sufficient concentrations of thyroid hormone must be present before birth and in early life for normal brain development, bone maturation, and production of correct amounts of growth hormone. Deficiencies can stunt growth and impair hearing, motor control, and intelligence in newborns and the young. Throughout life, thyroid hormones influence vertebrate energy demands by stimulating cells to burn more oxygen. A cell's oxygen use creates heat to moderate body temperature in warm-blooded animals and fuel metabolism (the body's ability to breakdown, store, and reclaim food releasing chemical energy in the process). Less than normal concentrations of thyroid hormone impairs oxygen and energy use while higher than normal concentrations over stimulates them. Seasonal changes such as fur molting in mammals or skin shedding by reptiles is directed by thyroid hormones. The hormones start hair growth in rodents, sheep, and other mammals. They also initiate amphibian metamorphosis that transforms tadpoles into frogs. A delicate balance of thyroid hormones ensures health in children and adults. Too much or too little shifts the status quo causing developmental problems, thyroid gland swelling (goiter), and a number of other health problems, including cancer. Childhood radiation exposure, living in areas with low or high iodine levels, consuming high levels of iodine, certain drugs, and endocrine disrupting compounds can alter thyroid hormone balance. An overabundance, called hyperthyroidism, manifests in weight loss, diarrhea, racing heart, and irritability. For example, goiter, bulging eyes, and skin problems occur in those with a type of hyperthyroidism known as Grave's disease. Not enough thyroid hormone, called hypothyroidism, may cause sudden weight gain, sluggishness, slow heart rate, dry skin, constipation, and coldness in adults. Human babies exposed to low or no thyroid hormones in the womb are born with short bones, weak muscles, and severe brain damage. Slow, stunted growth is common in children facing thyroid hormone deficiencies. back to topThyroid Disrupters Substances can disrupt nearly every part of thyroid hormone production, delivery, and breakdown. But, unlike estrogens, androgens, and other steroid hormones, they do not strongly interfere with thyroid receptors. Deciphering how endocrine disrupting compounds influence thyroid hormones is important due to their essential role in vertebrate brain development and energy-sustaining functions. click here to read more about wildlife effects... click here to read more about human effects... Many environmental compounds, including the pesticides 2,4-D and DDD and the industrial chemicals PCBs and dioxins, interfere with thyroid hormone processes (Howdeshell 2002). The substances can affect hormone production by altering iodide use and delivery. They can affect thyroid enzymes that put together or take apart the hormones. They can alters thyroid hormone delivery to body tissues by changing blood transfer protein concentrations. Some of the most studied thyroid disruptors focus on brain effects. PCBs changed hearing in rodent offspring and adults (Goldey and Crofton 1998). PCBs also increased thyroxine-altering enzyme action in fetal rodent brains and reduced its activity in female pups (Morse et al. 1993). Bisphenol-A, a ubiquitous product additive detected in human blood and fetal umbilical chord blood, binds to rat thyroid receptor and increases thyroxin blood concentrations in developing rat brains (Zoeller et al. 2004). Additionally, some PCBs and their breakdown products double-up on thyroid hormones. First, they can tie up thyroid transport proteins in the blood leaving natural thyroid hormones without an escort. Secondly, the compounds boost certain hormone destroying liver enzymes. In essence, the free-floating thyroid hormone is filtered from the blood into the liver, quickly coated with sugar, and excreted out of the body. Thyroid hormone levels fall, and the thyroid gland balloons into a goiter. Enlarged thyroid glands have been observed in wildlife, such as herring gulls and rainbow trout, living in the Great Lakes region (Moccia et al. 1986; Leatherland and Sonstegard 1980). back to topResearch History Human health and curiosity drive the need for broader understanding of science and biology. This is evident as early as the 1400 and 1500s when anatomists described the thyroid gland and realized that a goiter was simply an enlarged thyroid. More than three centuries passed before scientists in 1849 determined iodine deficiency caused goiter. It took another 100 years to fine tune what was known about the thyroid gland and its iodine-containing hormones. During that century, researchers busily pinpointed thyroid’s health effects and focused on understanding and identifying responsible hormones. Adolf Magnus-Levy (1895) took a first step in 1895 when he showed metabolic rate increased in patients who ate thyroid gland extract. This discovery linked the thyroid gland to the body’s ability to produce energy (heat). The following year, E. Baumann (1896) found iodine in the thyroid gland but not in other tissues—a unique and unexpected surprise (Rosenfeld 2000). Next, Edward C. Kendall (1915) isolated the thyroxine hormone in 1915, and Charles R. Harington (1926) synthesized it in 1926. It was finally realized by the mid-20th Century in 1952 that the hormone triiodothyronine (T3), not the higher concentration thyroxine (T4), was the most active form of thyroid hormone (Hadley 2000). The quest for a deeper understanding of thyroid hormones continues as endocrine disruption and other issues spur researchers to learn even more about how the thyroid maintains health and promotes disease. back to topReferences
back to top |