Endocrine Disruption
|
Actions : Docking :: Receptor Binding
Steroid Hormone Receptors Steroid hormones convey their molecular messages and give instructions by binding to proteins called receptors. There are five major categories of steroid hormone receptors in vertebrates: estrogen receptors (ER), androgen receptors (AR), progestin receptors (PR), glucocorticoid receptors (GR), and mineralocorticoid receptors (MR). Each group contains membrane, cytoplasmic, or nuclear receptors based on the receptor proteins location in the cell - outside in the plasma membrane, or inside in the cytoplasm or the nucleus. Like all proteins, receptors are assembled by ribosomes, the cell’s protein making machine. Ribosomes manufacture proteins following RNA (ribonucleic acid) instructions sent from the nucleus. Free-floating ribosomes make and deposit receptor proteins in the cytoplasm where they either stay or are transported into the nucleus. Ribosomes attached to the endoplasmic reticulum - called attached ribosomes - incorporate the receptors they make into the cell’s plasma membrane or package it for export. The most well studied steroid hormone receptors are those located inside the cell. Because the molecular structure and function of these receptors are the same whether they are in the cytoplasm or nucleus, these receptors are usually lumped together as nuclear receptors. General Structure Nuclear steroid hormone receptors are part of the nuclear receptor superfamily consisting of many receptor families, including steroid hormone receptors, thyroid hormone receptors, retinoic acid receptors, vitamin D receptors, peroxisome proliferator-activated receptors, pregnane X receptors, melatonin receptors (RZR), and many more. 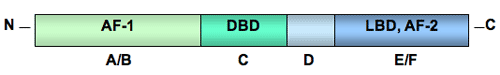 CAPTION: General structure of a nuclear steroid hormone receptor with five identified domains: the N-terminal, (A/B), the DNA binding domain or DBD (C), the hinge region (D), and the ligand-binding domain or LBD (E/F). CREDIT: Tulane University. All nuclear receptors share a common general structure with five structurally distinct sections called domains: the amino terminal (N-terminal) domain, the DNA binding domain (DBD), the hinge region, and the ligand-binding domain (LBD) (Mangelsdorf et al. 1995). The N-terminal domain contains activation function-1 (AF-1), a region necessary for ligand-independent interaction of the receptor with other proteins that stimulate gene transcription. The DBD contains two zinc fingers, loops of the receptor protein that interact with specific hormone response elements in the promoter regions of specific genes. In the carboxy terminus, the ligand-binding domain (LBD) binds the hormone and activation function-2 (AF-2), a second transcriptional activation domain, enhances ligand-dependent interaction of the receptor with another set of proteins necessary to start the process of gene expression. back to topMultiple Receptor Types Many steroid nuclear receptors exist as two or more versions. The number of versions varies among different vertebrate groups. For example, two versions of estrogen receptors, ERalpha and ERbeta, are known for mammals, birds, and probably reptiles. Fish, on the other hand, have three known receptors: ERalpha, ERbeta, and ERgamma (Hawkins et al. 2000; Nilsson et al. 2001). The different receptor forms are made from genetic blueprints in one of two ways. Production is guided either by single genes each making a receptor type, as is the case for estrogen receptor versions, or by variations of a single gene product processed in different ways during genetic transcription and translation. In the later case, certain regions of the receptor gene may be transcribed differently (referred to as alternative splicing) producing mRNAs with different sequences, and therefore, proteins with different amino acid sequences and functions. Or, during translation of mRNA, alternative start codons (sets of RNA bases that code for an amino acid) can produce different sized proteins with varied functions. Hormone receptor variants give rise to broader, yet more tissue-specific responses that are not possible with a single version. Tissues can contain a combination of receptor versions, as many do. Or tissues can have predominately one receptor version that dictates or allows for tissue-specific expression. This tissue specificity, based on differing versions of receptors, explains how one hormone can mediate many different kinds of responses in different types of tissues. In some cases, receptors counteract another’s responses with one receptor version suppressing the transcription-activating capacity of the other version, presumably preventing over activation by the stimulating receptor. Vertebrates most likely have multiple steroid receptors because, during the evolutionary past, the entire genome in the ancestor to the vertebrate lineage was duplicated (Durand 2003; Shimeld and Holland 2000; Thornton et al. 2003). Additional gene duplication events in the jawed vertebrate lineage - including certain fish, amphibians, reptiles, birds, and mammals - may have led to the diversification of estrogen receptors and 3-ketosteroid (progestins, corticosteroids, and androgens) receptors (Thornton 2001). back to topEstrogen Receptors In most vertebrates, there are two known versions of nuclear estrogen receptors: ERalpha and ERbeta. Each receptor type is produced from a separate gene and differs in structure, tissue location, and function. Differences in structure are very slight. For instance, the AF-1 differs between the two receptors, but the DNA binding domain is identical. The volume of the ligand-binding domain is slightly smaller in ERbeta, but most ligands bind the two receptors with similar affinity (strength). ERalpha and ERbeta coexist in many mammalian tissues, including the uterus, breast, pituitary, bone, cardiovascular system, and central nervous system. The notable exception is the prostate, which expresses mainly ERbeta. ERbeta is also more abundant than ERalpha in the lungs and ovary (Nilsson et al. 2001). ERalpha and ERbeta regulate the same genes, but ERalpha is usually more efficient at initiating transcription. When both ERs are present and bound to estrogen, ERbeta tends to inhibit ERalpha-stimulated transcription (McDonnell and Norris 2002). In fish, there is a third version of nuclear estrogen receptor, ERgamma, encoded by a separate gene. Sequence analysis indicates that ERgamma arose by duplication of the ERbeta gene in teleost (spiny-finned bony) fishes. Like ERbeta, ERgamma is abundant in the testis, but unlike ERalpha and beta, is also abundant in the ovary. ERgamma is minimally expressed in the liver. Interestingly, the three ERs have very distinct distributions in the hypothalamus, suggesting that each plays a unique role in regulation of reproduction (Hawkins et al. 2000). Ligands can be synthesized that bind to one human ER better than the other and selectively activate or inhibit only that ER. These kinds of ligands are called selective estrogen receptor modulators or SERMs. SERMs are being developed to treat breast cancer more effectively, to enhance bone density without the risk of reproductive tract cancers, and to provide the cardiovascular benefits of estrogen without cancer side effects. Genistein and coumestrol - types of flavonoid plant compounds called phytoestrogens - are sometimes referred to as SERMs because in artificial cell culture systems they bind to and activate ERbeta-driven gene expression much more strongly than they bind to or activate ERalpha (Mueller et al. 2003; Nilsson et al. 2001). Whether this difference in binding affinity has any physiological significance is uncertain (Nilsson et al. 2001). back to topAndrogen Receptors The number of nuclear androgen receptor (AR) versions varies among vertebrates. In humans, there may be two androgen receptor isoforms (types that differ slightly in amino acid sequence). The same gene codes for the two proteins, but AR-A appears to be an N-terminally shortened version of the major isoform AR-B. The tissue distribution and function of AR-A have not been investigated (Lee and Chang 2003). In some species of fish, there are two known types of AR that are encoded by two different genes, have two different patterns of tissue distribution (Ikeuchi et al. 2001), and have different binding affinities for ligands (Sperry and Thomas 1999a, 1999b). back to topProgestin Receptors The number of nuclear progestin receptor (PR) versions differs among species. At least one species of fish, the Japanese eel, has two distinct types of nuclear PR produced from two different genes (Ikeuchi et al. 2001). The two known isoforms of nuclear PR, called PR-A and PR-B, in humans, birds (?), and reptiles are produced from a single gene by initiating translation at different start codons. Similar tissues, primarily the breast, uterus, ovary, placenta, and brain, contain the two isoforms but their functional differences are unclear. PR-A may function as a repressor of PR-B (Drummond et al. 2002), similar to the suppressive action of ERbeta on ERalpha. back to topGlucocorticoid Receptors A single glucocorticoid receptor (GR) gene is found in all studied vertebrates. The gene encodes several known versions of nuclear GR with different structures, ligand-binding affinities, and functions. Alternative splicing of GR gene transcripts forms the three types of GRs found in vertebrates: GRalpha, GRbeta, and GRgamma. GRalpha can exist as two subtypes, GRalpha-A and GRalpha-B, which are created by the initiation of translation at different start codons on the mRNA. All three GR types are expressed in a wide variety of tissues, probably because glucocorticoids have a central role in regulating metabolism. GRalpha is the most effective stimulator of gene transcription in glucocorticoid-responsive genes, with GRgamma about half as effective. GRbeta functions as a negative regulator of GRalpha, forming a dimer with ligand-bound GRalpha and blocking GRalpha-stimulated transcription (Lu and Cidlowski 2004). back to topMineralocorticoid Receptors Like the GR, there is only one mineralocorticoid receptor (MR) gene. Alternative splicing during transcription produces the multiple isoforms of mRNA (Zennaro et al. 1997). At least two MR proteins are produced in humans: wild type MR and MRdelta5,6. MRdelta5,6 lacks both the hinge region and ligand-binding domain but has a functional DNA binding domain, so it can act as a transcription factor in the absence of hormone. Even so, in the presence of hormone, MRdelta5,6 enhances MR-mediated transcription. Both MRs are expressed in sodium-transporting epithelia (densely packed tissues) in the kidney and colon as well as in non-epithelial tissues such as heart, lung, lymphocytes, and hippocampus (Zennaro et al. 2001). back to topReferences
back to top |